Atoms and Basic Atomic Structure
Ionic bonding
An ionic bond is the electrostatic force of attraction between the positive and negative ions in a giant ionic lattice with 6:6 coordination (surrounding each positive ion are six negative ions and surrounding each negative ion are six positive ions). In an ionic bond electrons are transferred. HOWEVER, this isn’t the ‘bond’, the bond is the electrostatic force of attraction, not the transferred electron!
6:6 Coordination
Ionic bonds are between metals and non-metals.
Examples:
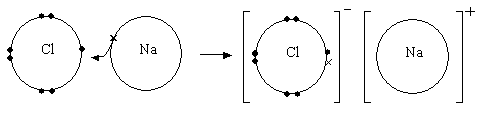
NaCl is an ionic bond – Sodium is in group 1 and to reach the stability
of the nearest noble gas it has to lose one electron. Chlorine is in group
7 and for it to reach the stability of the nearest noble gas it must gain
one electron. This results in an electron being transferred and the electrostatic
force of attraction that results from the positive sodium ions and the
negative chlorine ions holds the ions together in a giant ionic lattice
with 6:6 coordination.
Dot and cross diagrams
Ionic compound can be represented by dot and cross diagrams similar to covalent compounds, however in ionic compounds they are shown differently:
An example of a dot and cross diagram in a covalent compound (Outer Electrons only)
An example of a dot and cross diagram in an ionic compound
Melting/Boiling points in compounds
Ionic compounds have high melting/boiling points because of the strong electrostatic forces of attraction between the positive and negative ions in a giant ionic lattice.
Covalent compounds have low melting/boiling points because although the bonds between atoms are strong the electrostatic force of attraction between molecules is weak. It is these bonds that break when the compound is heated.
Ionic compound in water
When ionic compounds are dissolved in water the ions are attracted to
the water molecules separating them from the lattice and becoming hydrated.
Some ionic compounds, however won’t dissolve in water. This occurs if there is insufficient attraction to the water molecules to break the bonds. E.g. Na+ Cl- will dissolve in water, however Mg2+ O2- will not because MgO has stronger ionic bonds.
MgO also has a higher boiling point than NaCl because of the stronger
ionic bonds.
Ionic compounds and conductivity
Ionic compounds will not conduct electricity when solid as the ions are
not free to move, however when aqueous or liquid the ions are free to
move and it will therefore conduct electricity.
Test for ionic compounds
A simple test for an ionic compound is to see if it will conduct electricity when molten or aqueous.
Test for ions
Below are some basic chemical tests for ions. These do not have to be
learnt for the exam.
Test for cations
Cation Test If present colour of precipitate formed
Ca2+
Add NaOH Blue precipitate
Fe2+
Add NaOH Green precipitate
Fe3+
Add NaOH Brown precipitate
Ca2+
Add excess NaOH White precipitate forms that then doesn’t dissolve
when excess NaOH is used
Pb2+
Add excess NaOH White precipitate forms that then dissolves when excess
NaOH is used
Test for anions
Anion Test If present colour of precipitate formed
CO32-
Add HNO3 Blue precipitate
CO32-
Add HCl Fizzes
SO42-
Add Barium Chloride White precipitate
Cl- Add Silver Nitrate White precipitate
Br- Add Silver Nitrate Cream precipitate
I- Add Silver Nitrate Yellow precipitate
How to prepare an insoluble salt
Mix the two solutions, filter the mixture, wash the residue with distilled
water and then dry the residue in an oven until mass remains constant.
Example: Lead (II) nitrate solution with sodium iodide solution forms a precipitate of lead (II) iodide. This is filtered and the filtrate discarded is Sodium nitrate solution. The lead (II) iodide is then washed with distilled water and dried.
Soluble salts
This is where a salt is hydrated.
Hydrated compound
A compound that contains water of crystallisation. Water molecules form
part of the crystal lattice.
Equation for a hydrated salt
Na2CO3.10H2O
Sodium Carbonate Water of crystallisation
Anhydrous compound
A salt that’s left after water of crystallisation is driven off
the crystal lattice.
A precipitate
An insoluble solid formed when two solutions mix.
Isotopes
Definition
An atom of the same element with the same number of protons but a different
number of neutrons
Example:
Chlorine has two isotopes: 35Cl
and 37Cl
each with different percentage abundances. 75% of all Cl atoms have a
mass number of 35 and 25% have a mass number of 37. When calculating the
relative atomic mass of an element the percentage abundance of its isotopes
are used.
These are other definitions that you must learn for the exam:
Relative atomic mass (Ar)
Average mass of an elements atoms relative to 1/12th that of a carbon-12
atom.
Relative isotopic mass
Mass of an isotope relative to 1/12th of a carbon-12 atom.
Relative molecular mass (Mr)
Mass of its molecules relative to 1/12th of a carbon-12 atom.
Relative formula mass
Total mass of atoms in a formula relative to 1/12th of a Carbon-12 atom
E.g. From periodic table – H2O
= (2x1) + 16 = 18
Reference Tools
Dictionary
Thesaurus
Maps
Scientific Calculator
Periodic Table
Translator
Unit Conversion
Module 1
Useful Chemistry Resources
Atoms and Basic Atomic Structure
Mass Spectrometer
Moles
Covalent Bonding and Atomic Shapes
Electronegativity
Intermolecular Forces and Covalent Structure
Electronic Configuration
Ionisation Energy and Period 3 Trends
Oxidation Numbers and Ionic Equations
Chemical Reactions
Group 7 - Halides and Halogens
